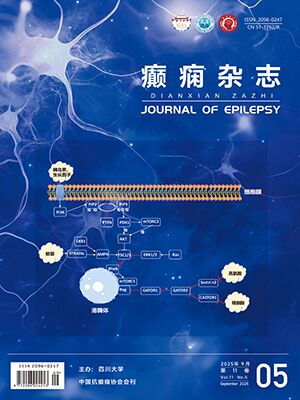
強烈應,
Email: qianglieying@126.com
癲癇是由于大腦神經元過度興奮,突然異常反復放電,導致大腦功能障礙所引發的臨床表現復雜多樣的慢性神經系統疾病。經藥物或手術治療后仍無法得到有效控制可發展為難治性癲癇,其機制復雜。不受控制的癲癇發作是危險的,不僅可導致認知、行為退化,還會增加死亡的風險。近年來關于難治性癲癇治療的研究已取得一定成果。本文將對近年來有關難治性癲癇的相關藥物和非藥物治療方法進行綜述,以期為臨床診治提供新思路。
Citation: 王新艷, 強烈應, 杜韞. 難治性癲癇:治療與展望. Journal of Epilepsy, 2023, 9(2): 128-133. doi: 10.7507/2096-0247.202208004 Copy
Copyright ? the editorial department of Journal of Epilepsy of West China Medical Publisher. All rights reserved
| 1. | WHO. Epilepsy Factsheet. WHO Webpage, 2020. |
| 2. | Beghi E, Giussani G, Nichols E, et al. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2019, 18(4): 357–375. |
| 3. | Desli E, Spilioti M, Evangeliou A, et al. The efficacy and safety of ketogenic diets in drug-resistant epilepsy in children and adolescents: a systematic review of randomized controlled trials. Current nutrition reports, 2022, 11(2): 102-116. |
| 4. | Beghi E, Camfield PR, Camfield CS. Epidemiologic aspects: lost intransition. Epilepsia, 2014, 55(Suppl 3): 3–7. |
| 5. | Gomez-Iba?ez A, McLachlan RS, Mirsattari SM, et al. Prognostic factors in patients with refractory idiopathic generalized epilepsy. Epilepsy Res, 2017, 130(2): 69-73. |
| 6. | Shuli L, Xing F, Ming Z, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma‐related epilepsy. Cancer Medicine, 2019, 8(10): 4527-4535. |
| 7. | Hwang ST, Stevens SJ, Fu AX, et al. Intractable generalized epilepsy: therapeutic approaches. Curr Neurol Neurosci Rep, 2019, 19(4): 16. |
| 8. | Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia, 2011, 52(11): 1956-1965. |
| 9. | Xin T, Qianyun C, Dezhi G, et al. Chinese expert recommendations on ketogenic diet therapy for super-refractory status epilepticus. Acta Epileptologica, 2022, 4(1): 1-11. |
| 10. | Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commissionfor classification and terminology. Epilepsia, 2017, 58(4): 522-530. |
| 11. | Lowenstein DH. Seizures and epilepsy(20th ed). Harrison’s Principles of Internal Medicine, 2018: 3050-3068. |
| 12. | L?scher W, Potschka H, Sisodiya SM, et al. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev, 2020, 72(3): 606-638. |
| 13. | Peng J, Pang N, Wang Y, et al. Next-generation sequencing improves treatment efficacy and reduces hospitalization in children with drug-resistant epilepsy. CNS Neurosci Ther, 2019, 25(1): 14-20. |
| 14. | Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr, 2016, 16(1): 48-61. |
| 15. | Vossler DG, Bainbridge JL, Boggs JG, et al. Treatment of refractory convulsive status epilepticus: a comprehensive review by the American Epilepsy Society Treatments Committee. Epilepsy Curr, 2020, 20(5): 245-264. |
| 16. | Eldufani J, Nekoui A, Blaise G. Non-anesthetic effects of ketamine. Journal of Health & Medical Informatics, 2018, 9(2): 1-6. |
| 17. | Alkhachroum, A, Der-Nigoghossian, C, Mathews, E, et al. Ketamine to treat super-refractory status epilepticus. Neurology, 2020, 95(16): e2286-e2294. |
| 18. | Rosati, A, De Masi, S, Guerrini, R. Ketamine for refractory status epilepticus: a systematic review. CNS Drugs, 2018, 32(11): 997-1009. |
| 19. | Petra Opi?, Raoul Sutter. The unease when using anesthetics for treatment-refractory status epilepticus: still far too many questions. Journal of Clinical Neurophysiology, 2020, 37(5): 399-405. |
| 20. | AG Marta, SF Ivan, MS Wainwright, et al. Novel drugs and early polypharmacotherapy in status epilepticus. Seizure, 2019, 68: 79-88. |
| 21. | Snoeren A, Majoie MHJM, Fasen KCFM, et al. Brivaracetam for the treatment of refractory epilepsy in patients with prior exposure to levetiracetam: a retrospective outcome analysis. Seizure, 2022, 96: 102-107. |
| 22. | Simona L, Cana-foglia L, Paola-Canevini M, et al. Adjunctive brivaracetam in older patients with focal seizures: evidence from the brivaracetam add-on first italian network study (BRIVAFIRST). Drugs & Aging, 2022, 39(4): 297-304. |
| 23. | Kouji F, Motohiro O. Brivaracetam and levetiracetam suppress astroglial l-glutamate release through hemichannel via inhibition of synaptic vesicle protein. International Journal of Molecular Sciences, 2022, 23(9): 4473-4473. |
| 24. | Nakamura M, Cho JH, Shin H, et al. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol, 2019, Jul,855: 175-182. |
| 25. | Zaccara G, Lattanzi S, Leo A, et al. Critical appraisal of cenobamate as adjunctive treatment of focal seizures in adults. Neuropsychiatric disease and treatment, 2021, 17: 3447-3457. |
| 26. | Steinhoff BJ, Rosenfeld WE, Serratosa JM, et al. Practical guidance for the management of adults receiving adjunctive cenobamate for the treatment of focal epilepsy-expert opinion. Epilepsy & Behavior, 2021, 123: 108270. |
| 27. | Reena E, Emily Z, Pamela C, et al. Cenobamate treatment of focal-onset seizures: Quality of life and outcome during up to eight years of treatment. Epilepsy & Behavior, 2021, 116: 107796. |
| 28. | Robin VT, Yash SD, Shefali K, et al. Adjunctive use of cenobamate for pediatric refractory focal-onset epilepsy: a single-center retrospective study. Epilepsy & Behavior, 2022, 130: 108679. |
| 29. | Amin MR, Ali DW. Pharmacology of medical cannabis. Springer Nature, 2019, 1162: 151-165. |
| 30. | Maria MB, Marta Z. Use of cannabidiol in the treatment of epilepsy. Neurologia I Neurochirurgia Polska, 2022, 56(1): 14-20. |
| 31. | Gray RA, Whalley BJ. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord, 2020, 22(S1): 10-15. |
| 32. | Klotz K A, Grob D, Sch?nberger J, et al. Effect of cannabidiol on interictal epileptiform activity and sleep architecture in children with intractable epilepsy: a prospective open-label study. CNS Drugs, 2021, 35(11): 1207-1215. |
| 33. | Caraballo R, Reyes G, Demirdjian G, et al. Long-term use of cannabidiol-enriched medical cannabis in a prospective cohort of children with drug-resistant developmental and epileptic encephalopathy. Seizure, 2022, 95: 56-63. |
| 34. | Harte S, Singh Y, Malone S, et al. Wallace G. Cannabidiol and refractory epilepsy:parental and caregiver perspectives of participation in a compassionate access scheme. BMC Health Services Research, 2022, 22(1): 173-173. |
| 35. | Brodie MJ, Ben-Menachem E. Cannabinoids for epilepsy: what do we know and where do we go? Epilepsia, 2018, 59(2): 291-296. |
| 36. | Von Wrede R, Helmstaedter C, Surges R. Cannabidiol in the treatment of epilepsy. Clinical Drug Investigation, 2021, 41(3): 211-220. |
| 37. | Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia, 2017, 58(9): 1586-1592. |
| 38. | Marchese F, Vari MS, Verrotti A, et al. An Open retrospective study of a standardized cannabidiol based-oil in treatment-resistant epilepsy. Cannabis and Cannabinoid Research, 2022, 7(2): 199-206. |
| 39. | Carla M, Maria G. Novel treatments in epilepsy guided by genetic diagnosis. British journal of clinical pharmacology, 2021, 88(6): 2539-2551. |
| 40. | Janmohamed M, Martin J, Kwan P. Pharmacoresistance – epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology, 2020, 168: 107790. |
| 41. | Pérez-Pérez D , Frías-Soria CL , Rocha L. Drug-resistant epilepsy: from multiple hypotheses to an integral explanation using preclinical resources. Epilepsy & Behavior, 2021, 121(Pt B): 106430. |
| 42. | Parker WE, Orlova KA, Parker WH, et al. Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sci Transl Med, 2013, 5(182): 53. |
| 43. | Gage M, Putra M, Wachter L, et al. Saracatinib, a src tyrosine kinase inhibitor, as a disease modifier in the rat dfp model: sex differences, neurobehavior, gliosis, neurodegeneration, and nitro-oxidative stress. Antioxidants, 2021, 11(1): 61. |
| 44. | 李欣, 陳嘉誠, 喬月朋, 等. 基于網絡藥理學的柴胡治療癲癇有效活性成分、作用靶基因篩選及靶基因生物學功能分析. 山東醫藥, 2022, 62(9): 57-62. |
| 45. | 周嬌嬌, 張青萍, 吳成挺, 等. “鉤藤 - 黃芩” 藥對治療癲癇的潛在作用機制探討. 中醫藥臨床雜志, 2022, 34(3): 498-504. |
| 46. | Yamanaka G, Ishida Y, Kanou K, et al. Towards a treatment for neuroinflammation in epilepsy: interleukin-1 receptor antagonist, anakinra, as a potential treatment in intractable epilepsy. International Journal of Molecular Sciences, 2021, 22(12): 1-12. |
| 47. | Dilena R, Mauri E, Aronica E, et al. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection–related epilepsy syndrome. Epilepsia Open, 2019, 4(2): 344-350. |
| 48. | Jun JS, Lee ST, Kim R, et al. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol, 2018, 84(6): 940-945. |
| 49. | Cantarín-Extremera V, Jiménez-Legido M, Duat-Rodríguez M, et al. Tocilizumab in pediatric refractory status epilepticus and acute epilepsy: experience in two patients. Journal of Neuroimmunology, 2020, 340: 577142. |
| 50. | Kossof EH, Zupec-Kania BA, Auvin S, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open, 2018, 3(2): 175-192. |
| 51. | Barry D, Ellul S, Watters L, et al. The ketoenic diet in disease and development. International Journal of Developmental Neuroscience, 2018, 68: 53-58. |
| 52. | Dressler A, Trimmel-Schwahofer P. The ketogenic diet for infants: How long can you go? Epilepsy Res, 2020, 164: 106339. |
| 53. | Wells J, Swaminathan A, Paseka J, et al. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy—a review. Nutrients, 2020, 12(6): 1809. |
| 54. | Xiangjun D, Xiaoke X, Tingting M, et al. Evaluation of the seizure control and the tolerability of ketogenic diet in Chinese children with structural drug-resistant epilepsy. Seizure, 2022, 94: 43-51. |
| 55. | 方雨. 生酮飲食在基因突變所致難治性癲癇中應用及療效的研究進展. 國際兒科學雜志, 2022, 49(1): 39-43. |
| 56. | Paketci C, Edem P, Hiz S, et al. Successful treatment of intractable epilepsy with ketogenic diet therapy in twins with ALG3 -CDG. Brain and Development, 2020, 42(7): 539-545. |
| 57. | Kaul N, Nation J, Laing J, et al. Modified low ratio ketogenic therapy in the treatment of adults with super refractory status epilepticus. Journal of Parenteral and Enteral Nutrition, 2022, 13: 1-9. |
| 58. | 陳婷, 陳婭, 張海青, 等. 腸道微生態與癲癇的相關性研究進展. 中華神經醫學志, 2019, 18(4): 413-416. |
| 59. | Arulsamy A, Tan QY, Balasubramaniam V, et al. Gut microbiota and epilepsy: a systematic review on their relationship and possible therapeutics. ACS Chem Neurosci, 2020, 11(21): 3488-3498. |
| 60. | Mengoni F, Salari V, Kosenkova I, et al, Gut microbiota modulates seizure susceptibility. Epilepsia, 2021 , 62(9): e153-e157. |
| 61. | K?z?laslan N, Sumbul O, Aygun H. The benefcial efect of probiotics supplementation on penicillin-induced focal seizure in rats. Neurochemical Research, 2022, 47(5): 1395-1404. |
| 62. | Holmes M, Flaminio Z, Vardhan M, et al. Cross talk between drug-resistant epilepsy and the gut microbiome. Epilepsia, 2020, 61(12): 2619-2628. |
| 63. | Olson CA, I?iguez AJ, Yang GE, et al. Alterations in the gut microbiota contribute to cognitive impairment induced by the ketogenic diet and hypoxia. Cell Host & Microbe, 2021, 29(9): 1378-1392. |
- 1. WHO. Epilepsy Factsheet. WHO Webpage, 2020.
- 2. Beghi E, Giussani G, Nichols E, et al. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2019, 18(4): 357–375.
- 3. Desli E, Spilioti M, Evangeliou A, et al. The efficacy and safety of ketogenic diets in drug-resistant epilepsy in children and adolescents: a systematic review of randomized controlled trials. Current nutrition reports, 2022, 11(2): 102-116.
- 4. Beghi E, Camfield PR, Camfield CS. Epidemiologic aspects: lost intransition. Epilepsia, 2014, 55(Suppl 3): 3–7.
- 5. Gomez-Iba?ez A, McLachlan RS, Mirsattari SM, et al. Prognostic factors in patients with refractory idiopathic generalized epilepsy. Epilepsy Res, 2017, 130(2): 69-73.
- 6. Shuli L, Xing F, Ming Z, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma‐related epilepsy. Cancer Medicine, 2019, 8(10): 4527-4535.
- 7. Hwang ST, Stevens SJ, Fu AX, et al. Intractable generalized epilepsy: therapeutic approaches. Curr Neurol Neurosci Rep, 2019, 19(4): 16.
- 8. Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia, 2011, 52(11): 1956-1965.
- 9. Xin T, Qianyun C, Dezhi G, et al. Chinese expert recommendations on ketogenic diet therapy for super-refractory status epilepticus. Acta Epileptologica, 2022, 4(1): 1-11.
- 10. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commissionfor classification and terminology. Epilepsia, 2017, 58(4): 522-530.
- 11. Lowenstein DH. Seizures and epilepsy(20th ed). Harrison’s Principles of Internal Medicine, 2018: 3050-3068.
- 12. L?scher W, Potschka H, Sisodiya SM, et al. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev, 2020, 72(3): 606-638.
- 13. Peng J, Pang N, Wang Y, et al. Next-generation sequencing improves treatment efficacy and reduces hospitalization in children with drug-resistant epilepsy. CNS Neurosci Ther, 2019, 25(1): 14-20.
- 14. Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr, 2016, 16(1): 48-61.
- 15. Vossler DG, Bainbridge JL, Boggs JG, et al. Treatment of refractory convulsive status epilepticus: a comprehensive review by the American Epilepsy Society Treatments Committee. Epilepsy Curr, 2020, 20(5): 245-264.
- 16. Eldufani J, Nekoui A, Blaise G. Non-anesthetic effects of ketamine. Journal of Health & Medical Informatics, 2018, 9(2): 1-6.
- 17. Alkhachroum, A, Der-Nigoghossian, C, Mathews, E, et al. Ketamine to treat super-refractory status epilepticus. Neurology, 2020, 95(16): e2286-e2294.
- 18. Rosati, A, De Masi, S, Guerrini, R. Ketamine for refractory status epilepticus: a systematic review. CNS Drugs, 2018, 32(11): 997-1009.
- 19. Petra Opi?, Raoul Sutter. The unease when using anesthetics for treatment-refractory status epilepticus: still far too many questions. Journal of Clinical Neurophysiology, 2020, 37(5): 399-405.
- 20. AG Marta, SF Ivan, MS Wainwright, et al. Novel drugs and early polypharmacotherapy in status epilepticus. Seizure, 2019, 68: 79-88.
- 21. Snoeren A, Majoie MHJM, Fasen KCFM, et al. Brivaracetam for the treatment of refractory epilepsy in patients with prior exposure to levetiracetam: a retrospective outcome analysis. Seizure, 2022, 96: 102-107.
- 22. Simona L, Cana-foglia L, Paola-Canevini M, et al. Adjunctive brivaracetam in older patients with focal seizures: evidence from the brivaracetam add-on first italian network study (BRIVAFIRST). Drugs & Aging, 2022, 39(4): 297-304.
- 23. Kouji F, Motohiro O. Brivaracetam and levetiracetam suppress astroglial l-glutamate release through hemichannel via inhibition of synaptic vesicle protein. International Journal of Molecular Sciences, 2022, 23(9): 4473-4473.
- 24. Nakamura M, Cho JH, Shin H, et al. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol, 2019, Jul,855: 175-182.
- 25. Zaccara G, Lattanzi S, Leo A, et al. Critical appraisal of cenobamate as adjunctive treatment of focal seizures in adults. Neuropsychiatric disease and treatment, 2021, 17: 3447-3457.
- 26. Steinhoff BJ, Rosenfeld WE, Serratosa JM, et al. Practical guidance for the management of adults receiving adjunctive cenobamate for the treatment of focal epilepsy-expert opinion. Epilepsy & Behavior, 2021, 123: 108270.
- 27. Reena E, Emily Z, Pamela C, et al. Cenobamate treatment of focal-onset seizures: Quality of life and outcome during up to eight years of treatment. Epilepsy & Behavior, 2021, 116: 107796.
- 28. Robin VT, Yash SD, Shefali K, et al. Adjunctive use of cenobamate for pediatric refractory focal-onset epilepsy: a single-center retrospective study. Epilepsy & Behavior, 2022, 130: 108679.
- 29. Amin MR, Ali DW. Pharmacology of medical cannabis. Springer Nature, 2019, 1162: 151-165.
- 30. Maria MB, Marta Z. Use of cannabidiol in the treatment of epilepsy. Neurologia I Neurochirurgia Polska, 2022, 56(1): 14-20.
- 31. Gray RA, Whalley BJ. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord, 2020, 22(S1): 10-15.
- 32. Klotz K A, Grob D, Sch?nberger J, et al. Effect of cannabidiol on interictal epileptiform activity and sleep architecture in children with intractable epilepsy: a prospective open-label study. CNS Drugs, 2021, 35(11): 1207-1215.
- 33. Caraballo R, Reyes G, Demirdjian G, et al. Long-term use of cannabidiol-enriched medical cannabis in a prospective cohort of children with drug-resistant developmental and epileptic encephalopathy. Seizure, 2022, 95: 56-63.
- 34. Harte S, Singh Y, Malone S, et al. Wallace G. Cannabidiol and refractory epilepsy:parental and caregiver perspectives of participation in a compassionate access scheme. BMC Health Services Research, 2022, 22(1): 173-173.
- 35. Brodie MJ, Ben-Menachem E. Cannabinoids for epilepsy: what do we know and where do we go? Epilepsia, 2018, 59(2): 291-296.
- 36. Von Wrede R, Helmstaedter C, Surges R. Cannabidiol in the treatment of epilepsy. Clinical Drug Investigation, 2021, 41(3): 211-220.
- 37. Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia, 2017, 58(9): 1586-1592.
- 38. Marchese F, Vari MS, Verrotti A, et al. An Open retrospective study of a standardized cannabidiol based-oil in treatment-resistant epilepsy. Cannabis and Cannabinoid Research, 2022, 7(2): 199-206.
- 39. Carla M, Maria G. Novel treatments in epilepsy guided by genetic diagnosis. British journal of clinical pharmacology, 2021, 88(6): 2539-2551.
- 40. Janmohamed M, Martin J, Kwan P. Pharmacoresistance – epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology, 2020, 168: 107790.
- 41. Pérez-Pérez D , Frías-Soria CL , Rocha L. Drug-resistant epilepsy: from multiple hypotheses to an integral explanation using preclinical resources. Epilepsy & Behavior, 2021, 121(Pt B): 106430.
- 42. Parker WE, Orlova KA, Parker WH, et al. Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sci Transl Med, 2013, 5(182): 53.
- 43. Gage M, Putra M, Wachter L, et al. Saracatinib, a src tyrosine kinase inhibitor, as a disease modifier in the rat dfp model: sex differences, neurobehavior, gliosis, neurodegeneration, and nitro-oxidative stress. Antioxidants, 2021, 11(1): 61.
- 44. 李欣, 陳嘉誠, 喬月朋, 等. 基于網絡藥理學的柴胡治療癲癇有效活性成分、作用靶基因篩選及靶基因生物學功能分析. 山東醫藥, 2022, 62(9): 57-62.
- 45. 周嬌嬌, 張青萍, 吳成挺, 等. “鉤藤 - 黃芩” 藥對治療癲癇的潛在作用機制探討. 中醫藥臨床雜志, 2022, 34(3): 498-504.
- 46. Yamanaka G, Ishida Y, Kanou K, et al. Towards a treatment for neuroinflammation in epilepsy: interleukin-1 receptor antagonist, anakinra, as a potential treatment in intractable epilepsy. International Journal of Molecular Sciences, 2021, 22(12): 1-12.
- 47. Dilena R, Mauri E, Aronica E, et al. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection–related epilepsy syndrome. Epilepsia Open, 2019, 4(2): 344-350.
- 48. Jun JS, Lee ST, Kim R, et al. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol, 2018, 84(6): 940-945.
- 49. Cantarín-Extremera V, Jiménez-Legido M, Duat-Rodríguez M, et al. Tocilizumab in pediatric refractory status epilepticus and acute epilepsy: experience in two patients. Journal of Neuroimmunology, 2020, 340: 577142.
- 50. Kossof EH, Zupec-Kania BA, Auvin S, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open, 2018, 3(2): 175-192.
- 51. Barry D, Ellul S, Watters L, et al. The ketoenic diet in disease and development. International Journal of Developmental Neuroscience, 2018, 68: 53-58.
- 52. Dressler A, Trimmel-Schwahofer P. The ketogenic diet for infants: How long can you go? Epilepsy Res, 2020, 164: 106339.
- 53. Wells J, Swaminathan A, Paseka J, et al. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy—a review. Nutrients, 2020, 12(6): 1809.
- 54. Xiangjun D, Xiaoke X, Tingting M, et al. Evaluation of the seizure control and the tolerability of ketogenic diet in Chinese children with structural drug-resistant epilepsy. Seizure, 2022, 94: 43-51.
- 55. 方雨. 生酮飲食在基因突變所致難治性癲癇中應用及療效的研究進展. 國際兒科學雜志, 2022, 49(1): 39-43.
- 56. Paketci C, Edem P, Hiz S, et al. Successful treatment of intractable epilepsy with ketogenic diet therapy in twins with ALG3 -CDG. Brain and Development, 2020, 42(7): 539-545.
- 57. Kaul N, Nation J, Laing J, et al. Modified low ratio ketogenic therapy in the treatment of adults with super refractory status epilepticus. Journal of Parenteral and Enteral Nutrition, 2022, 13: 1-9.
- 58. 陳婷, 陳婭, 張海青, 等. 腸道微生態與癲癇的相關性研究進展. 中華神經醫學志, 2019, 18(4): 413-416.
- 59. Arulsamy A, Tan QY, Balasubramaniam V, et al. Gut microbiota and epilepsy: a systematic review on their relationship and possible therapeutics. ACS Chem Neurosci, 2020, 11(21): 3488-3498.
- 60. Mengoni F, Salari V, Kosenkova I, et al, Gut microbiota modulates seizure susceptibility. Epilepsia, 2021 , 62(9): e153-e157.
- 61. K?z?laslan N, Sumbul O, Aygun H. The benefcial efect of probiotics supplementation on penicillin-induced focal seizure in rats. Neurochemical Research, 2022, 47(5): 1395-1404.
- 62. Holmes M, Flaminio Z, Vardhan M, et al. Cross talk between drug-resistant epilepsy and the gut microbiome. Epilepsia, 2020, 61(12): 2619-2628.
- 63. Olson CA, I?iguez AJ, Yang GE, et al. Alterations in the gut microbiota contribute to cognitive impairment induced by the ketogenic diet and hypoxia. Cell Host & Microbe, 2021, 29(9): 1378-1392.
-
Previous Article
結節性硬化精準治療與隨訪研究進展 -
Next Article
頂葉外側癲癇—解剖、生理與臨床